42 phase labels in chemical equations
Answered: Complete and balance the three… | bartleby Solution for Complete and balance the three equations according to acid and base behavior in water. Phase labels are optional. HNO, (aq) + H,O(1) H,0 Ba(ОН),… Phase plane - Wikipedia Phase plane. In applied mathematics, in particular the context of nonlinear system analysis, a phase plane is a visual display of certain characteristics of certain kinds of differential equations; a coordinate plane with axes being the values of the two state variables, say ( x, y ), or ( q, p) etc. (any pair of variables).
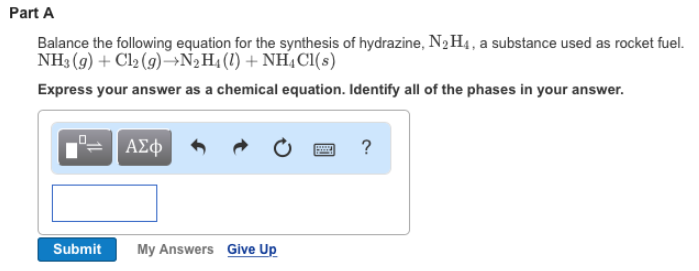
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.

Phase labels in chemical equations
Chemical Formula Labels Teaching Resources | Teachers Pay Teachers Browse chemical formula labels resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources. Writing and Balancing Chemical Equations - Chemistry 2e Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen (N 2) and oxygen (O 2) to form dinitrogen pentoxide. Solution First, write the unbalanced equation. Next, count the number of each type of atom present in the unbalanced equation. The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
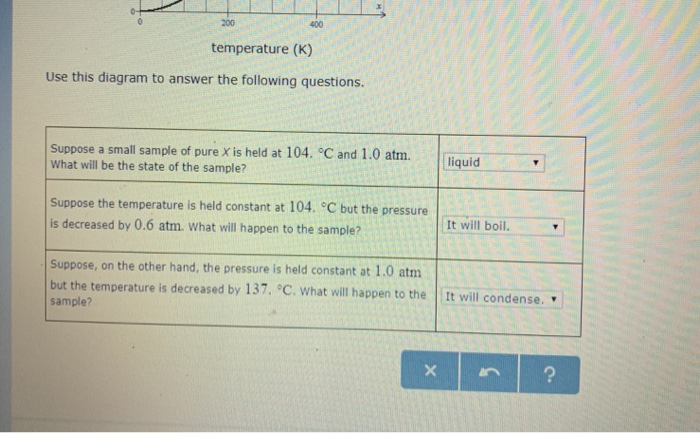
Phase labels in chemical equations. Many chemical equations also include phase labels for Many chemical equations also include phase labels for. School Eastern Visayas State University - Tacloban City Main Campus; Course Title AGRICULTUR 847,272; Uploaded By MasterPencil4687. Pages 46 This preview shows page 25 - 27 out of 46 pages. ... Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ)
Phase Definition and Examples - ThoughtCo In chemistry and physics, a phase is a physically distinctive form of matter, such as a solid, liquid, gas, or plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways Net Ionic Equations | Chemistry for Non-Majors | | Course Hero For example, there are six chloride ions on the reactant side because the coefficient of 3 is multiplied by the subscript of 2 in the copper (II) chloride formula. The spectator ions are K + and Cl − and can be eliminated. Net ionic equation: Step 3: Think about your result. For a precipitation reaction, the net ionic equation always shows ... Factor-Label Method in Chemistry: Definition, Examples & Practice ... The Factor-Label Method. Believe it or not, one simple method can be used to accomplish many of the basic calculations in chemistry. The method does not involve years of calculus courses or other ...
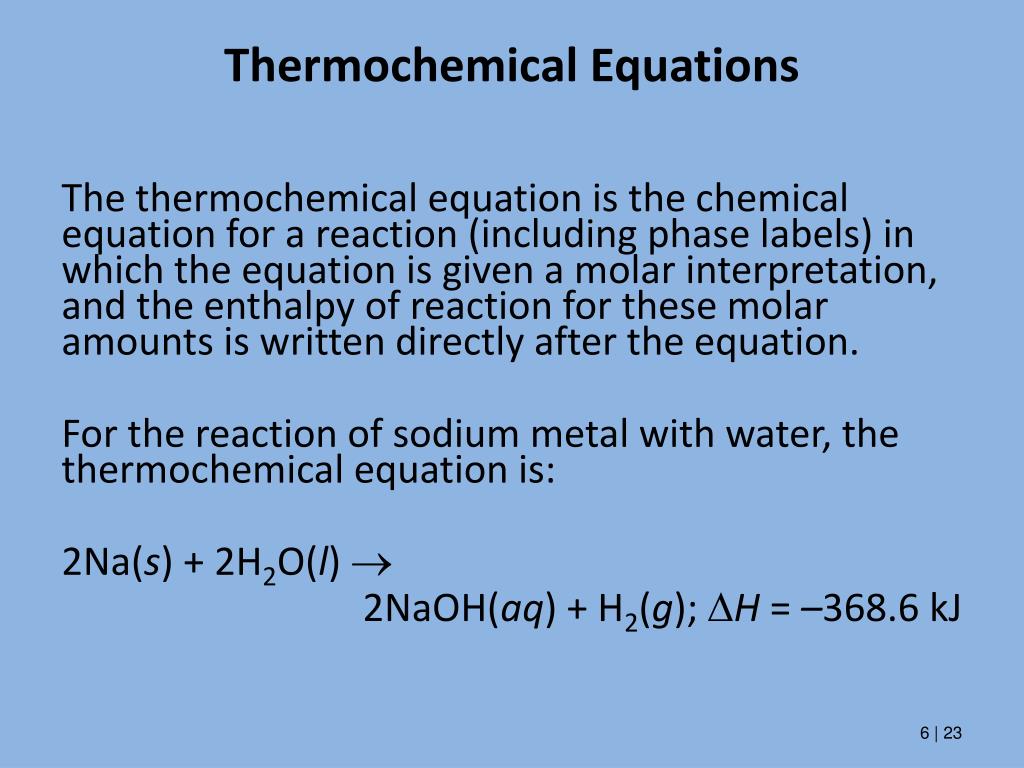
A thermochemical equation is a balanced chemical reaction equation ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS A reaction that produces heat is referred to as exothermic. For exothermic reactions, enthalpy values are assigned a negative value (-DH). Noting the phase labels, write the $K_{\text {sp }}$ expression for the ... everyone my name is now. I would answer the question right. The K is be expression forth the phone wing compound is that for the 1st 2 compound is, um Barrier Selfie burn himself fate when we dissolve it in water or not all pearl himself in the salt, only small amount off Burnham Selfie dissolved. And the's This salt is represented by case Be well in the soft it comforted toe It's I'm which is ... Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... 3 Steps for Balancing Chemical Equations - ThoughtCo A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substances), the formulas of the participants, the phases of the participants (solid, liquid, gas), the direction of the chemical reaction, and the amount of each substance. Chemical equations are balanced for mass and charge, meaning the number and type of atoms on the left side of the arrow is the same as the number of type of atoms on the ...
Chemical Equations - GitHub Pages It is not uncommon to include a phase label with each formula— (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H2(g) + O2(g) → H2O (ℓ) Note
Solved 1. Write balanced chemical equations using proper | Chegg.com Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron(III) oxide, also known as rust. b) Solid calcium carbonate is added to a solution of hydrochloric acid, yielding an aqueous solution of calcium chloride, water, and carbon ...

Point To Point Diagrams - 2 4 Phase Diagrams Chemistry Libretexts / Both numbers tell us about ...
What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used.
Chem - States of Matter in a Chemical Equation - Scientific Tutor How are states of matter displayed in a chemical equation? In this section we are going to show you how states of matter are displayed in a chemical equation. The states of matter are gas, like the air we breath, liquid, like the water we drink, solid, like the ground we are standing on, and aqueous like the sugary drink we taste.. The notation for each state is as follows below:
Using state symbols in chemical equations - BBC Bitesize Learn about and revise equations and chemical reactions with this BBC Bitesize GCSE Combined Science (OCR 21C) study guide.
How to Identify States of Matter in a Chemical Formula 1 Locate the parentheses after the chemical formula, whether or not it is within the context of an equation. 2 Identify the parentheses as (s) for solid, (l) for liquid, (g) for gas, or (aq) for...
Symbols in Chemical Equations - Harper College Symbol: Meaning + used to separate one reactant or product from another used to separate the reactants from the products - it is pronounced "yields" or "produces" when the equation is read
GCSE CHEMISTRY - What are State Symbols? - (s) - (l) - (g) - (aq ... The complete equations in words and symbols would be. potassium + chlorine potassium chloride. 2 K (s) + Cl 2(g) 2 K Cl (s) lith ium + oxygen lithium oxide. 4 Li (s) + O 2 (g) 2 Li 2 O (s) The state symbols in brackets show the physical state of. the substance at the reaction temperature.
The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
Writing and Balancing Chemical Equations - Chemistry 2e Balancing Chemical Equations Write a balanced equation for the reaction of molecular nitrogen (N 2) and oxygen (O 2) to form dinitrogen pentoxide. Solution First, write the unbalanced equation. Next, count the number of each type of atom present in the unbalanced equation.
Chemical Formula Labels Teaching Resources | Teachers Pay Teachers Browse chemical formula labels resources on Teachers Pay Teachers, a marketplace trusted by millions of teachers for original educational resources.









Post a Comment for "42 phase labels in chemical equations"